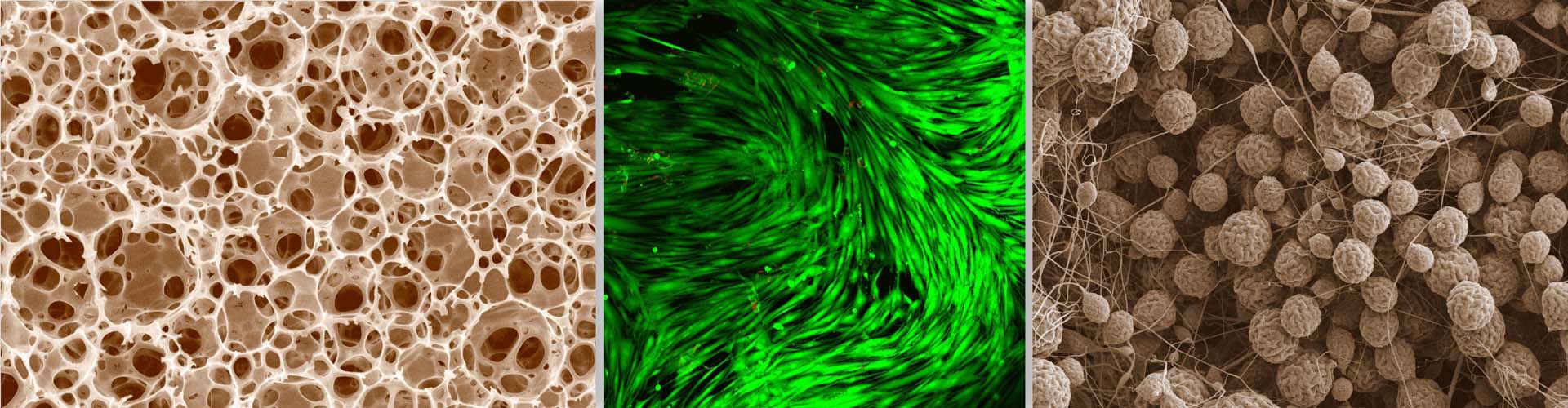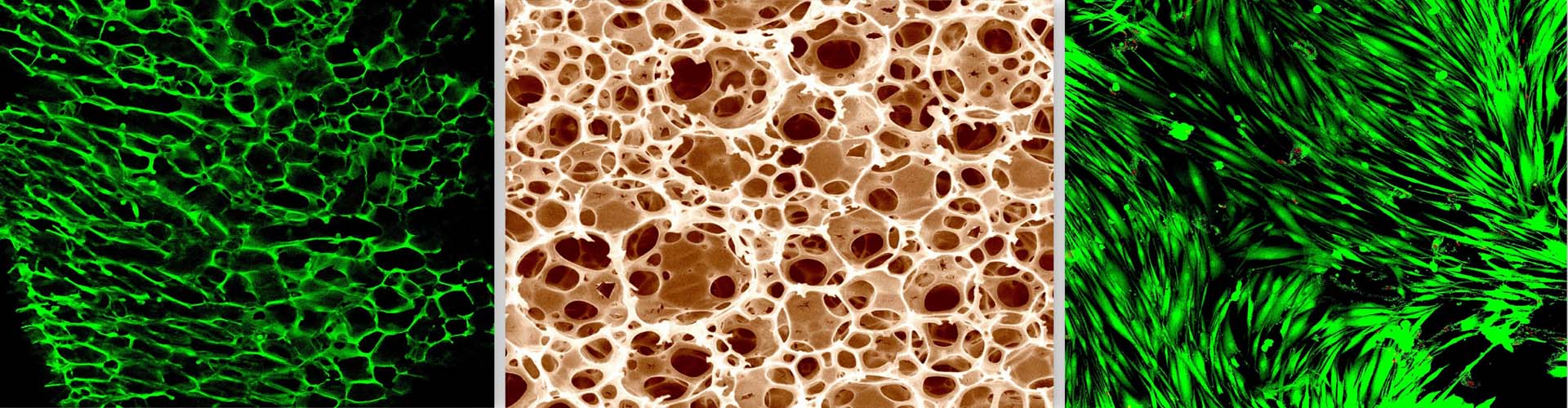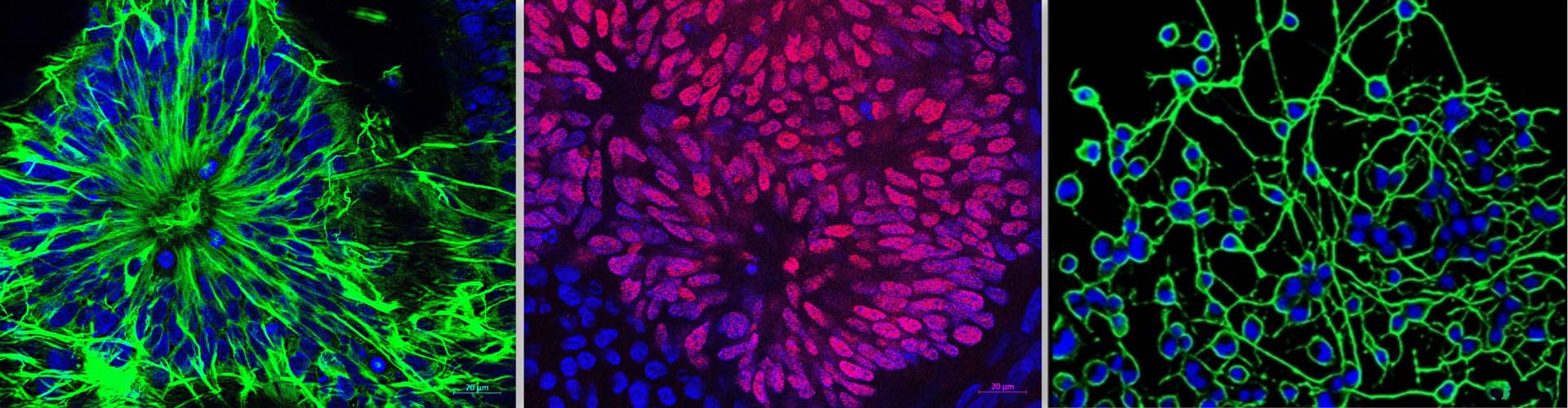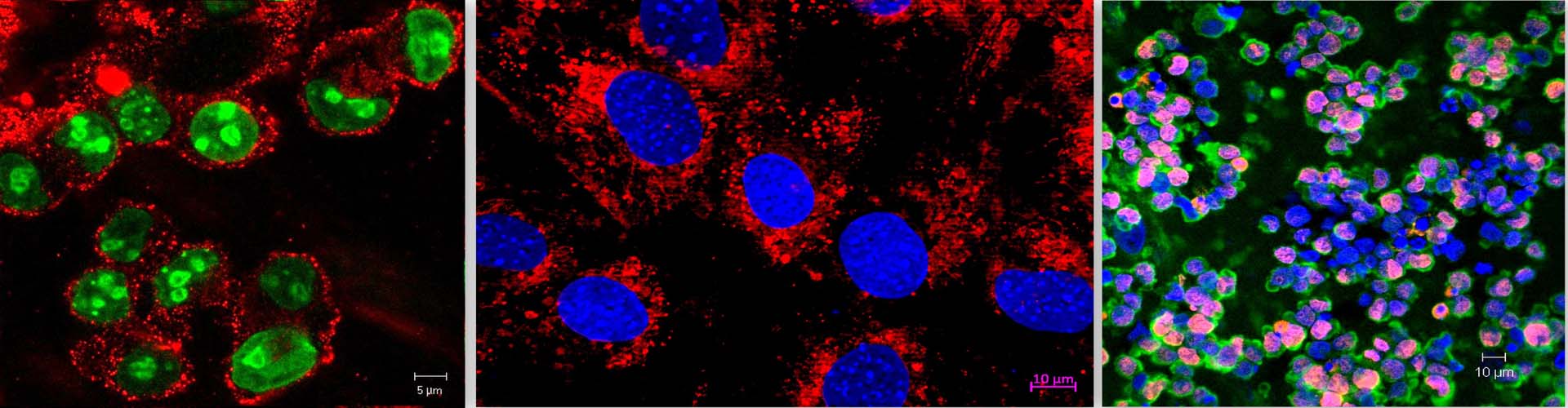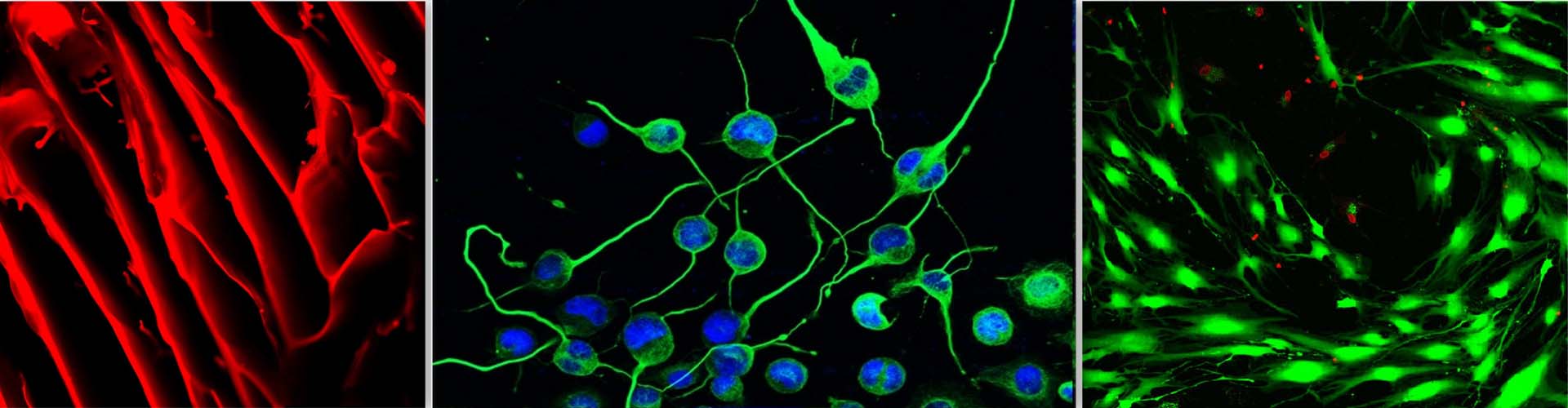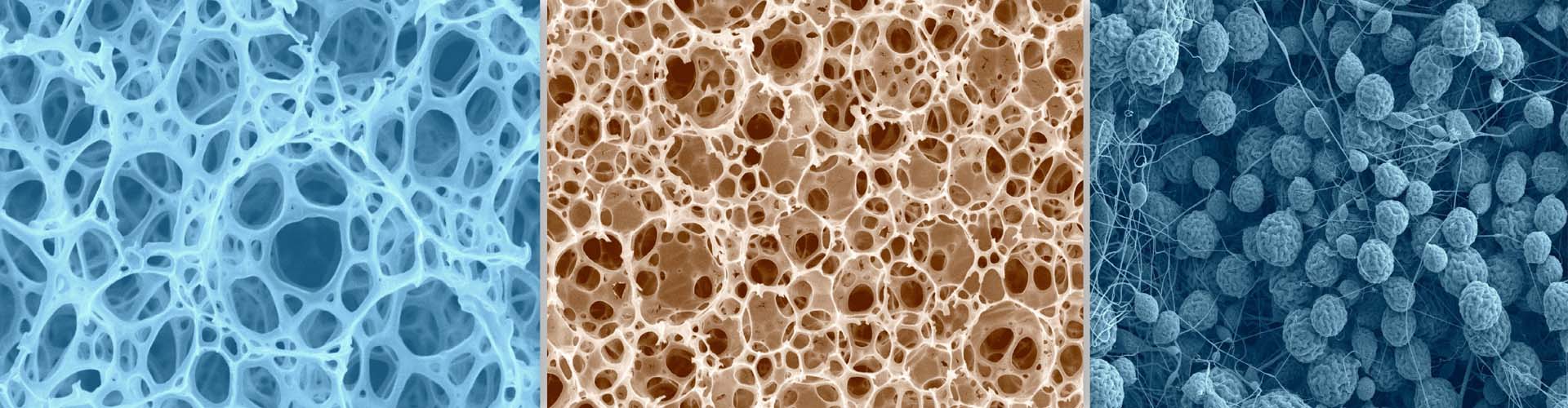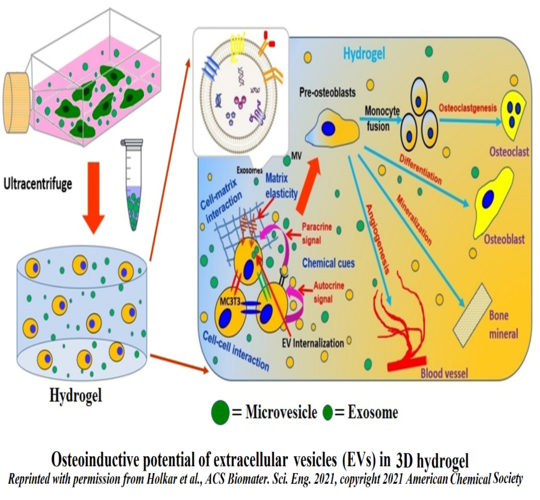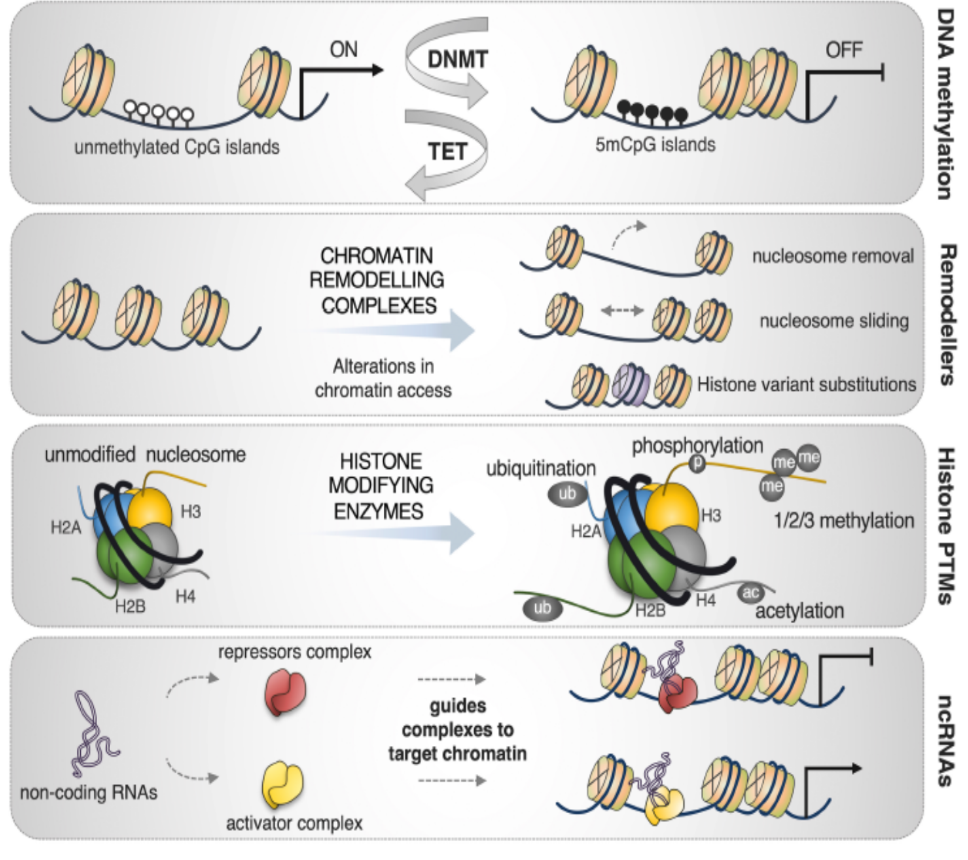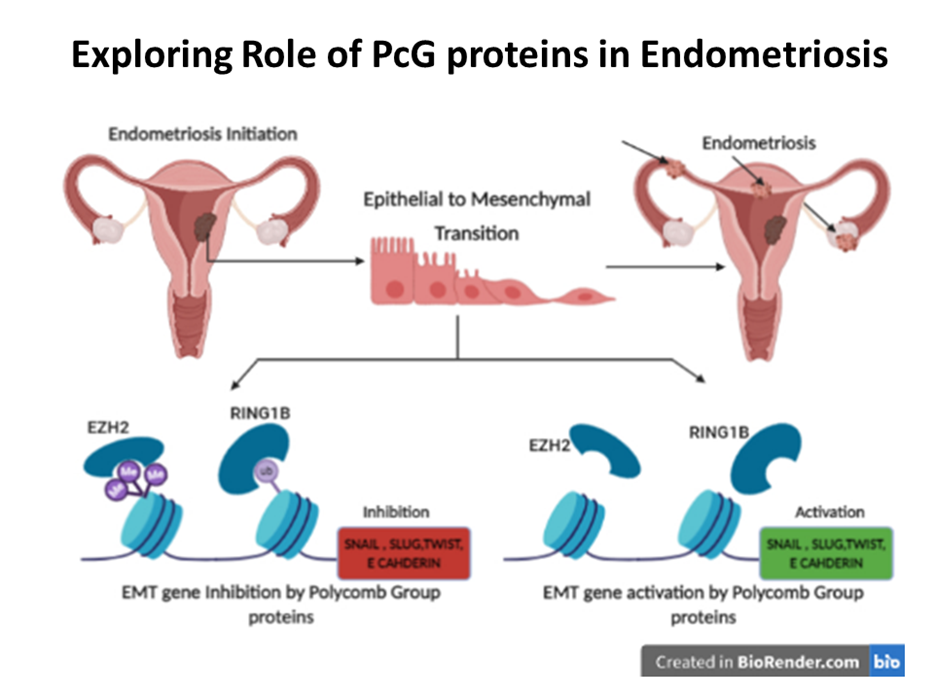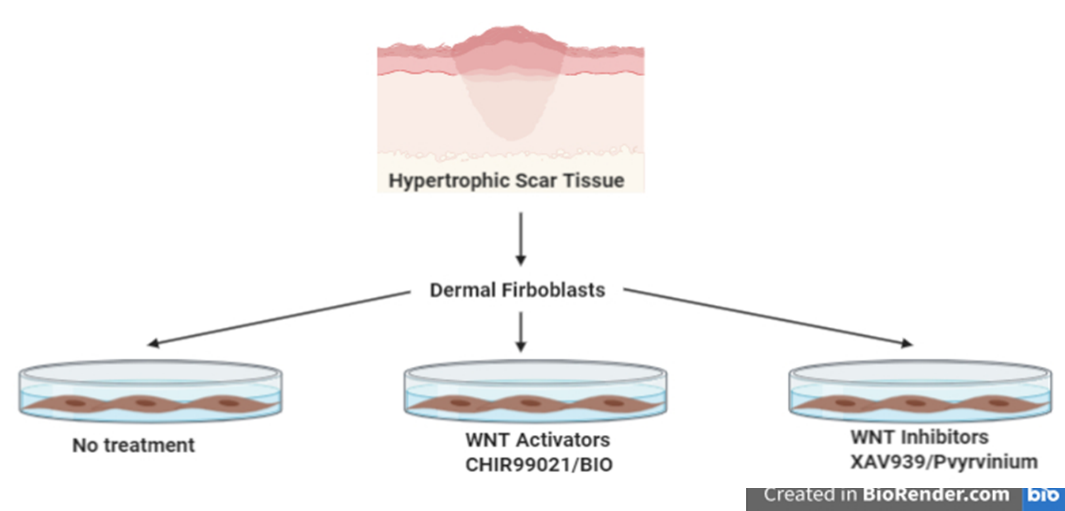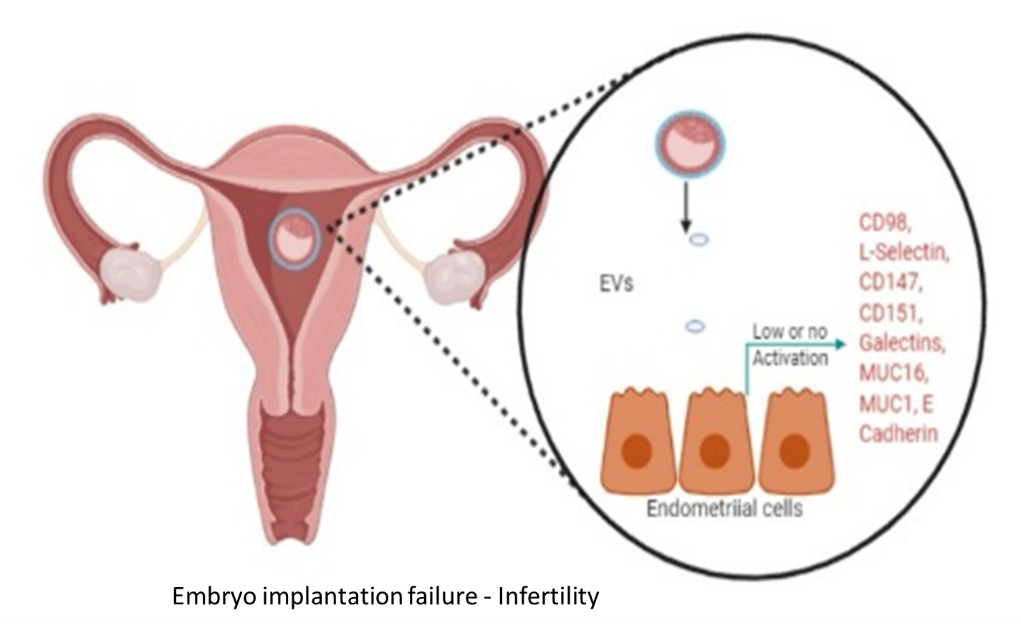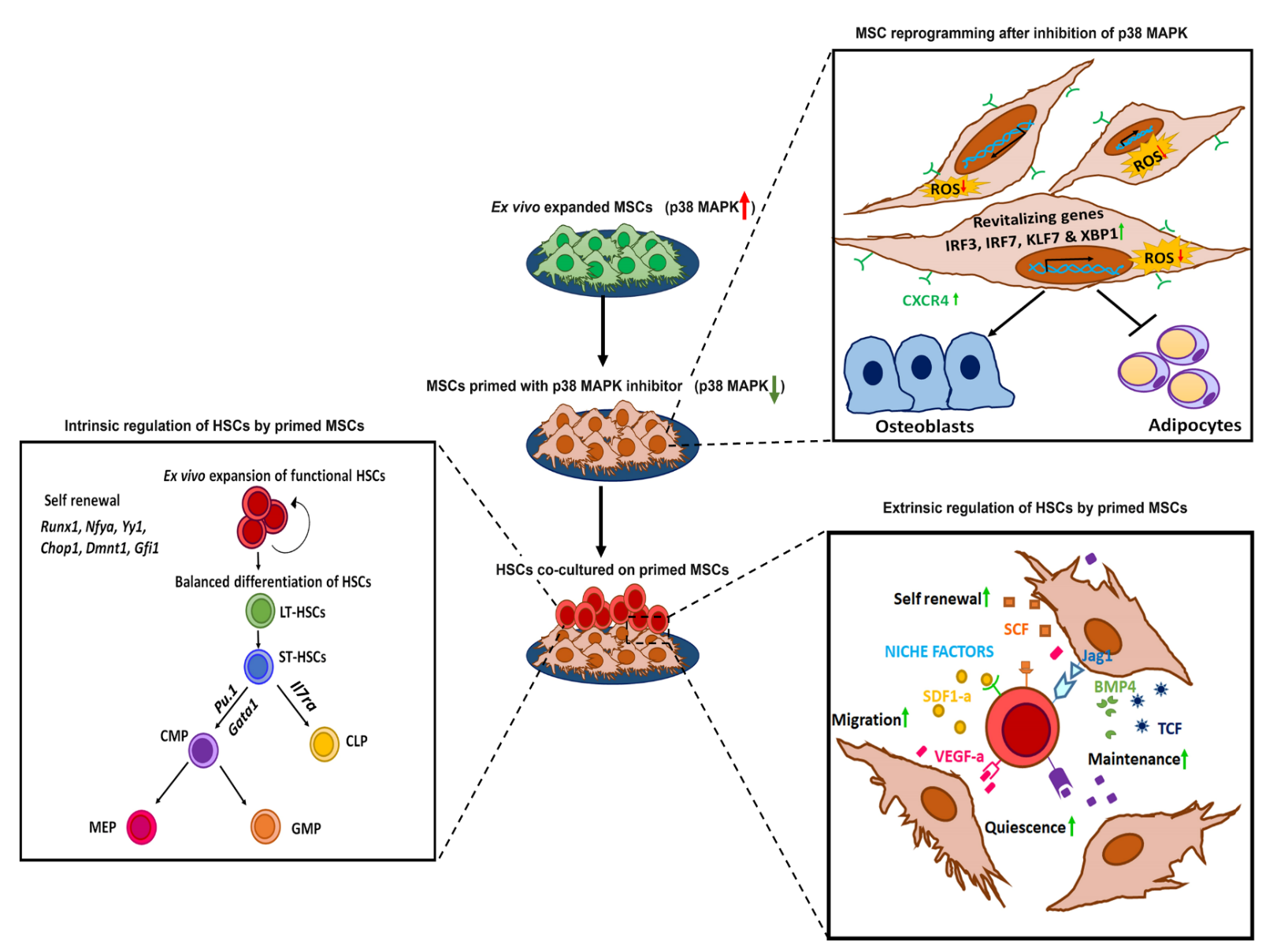
In the bone marrow microenvironment, the mesenchymal stromal cells (MSCs) respond to the external cues and modulate the fate of hematopoietic stem cells (HSCs) via the extracellular vesicles (EVs). The EVs viz. micro-vesicles (MVs) and exosomes, therefore, form an important extrinsic mechanism through which the stromal cells regulate the fate of HSCs.
In this project, we use a strategy known as “priming of MSCs” wherein we modulate the signalling pathways in MSCs, which improves their HSC- supportive ability. Such a strategy could yield a sufficient number of HSCs with superior functionality to overcome challenges associated with hematopoietic stem cell transplantation (HSCT) at the clinical level. Furthermore, priming also rejuvenates the MSCs, thereby improving the overall outcome of in vitro cultured MSCs for regenerative therapies. The EVs isolated from primed MSCs could also be used as off-the-shelf, cell-free biologics in a clinical setup.
Principal Investigator: Dr. Anuradha Vaidya and Dr. Vaijayanti Kale
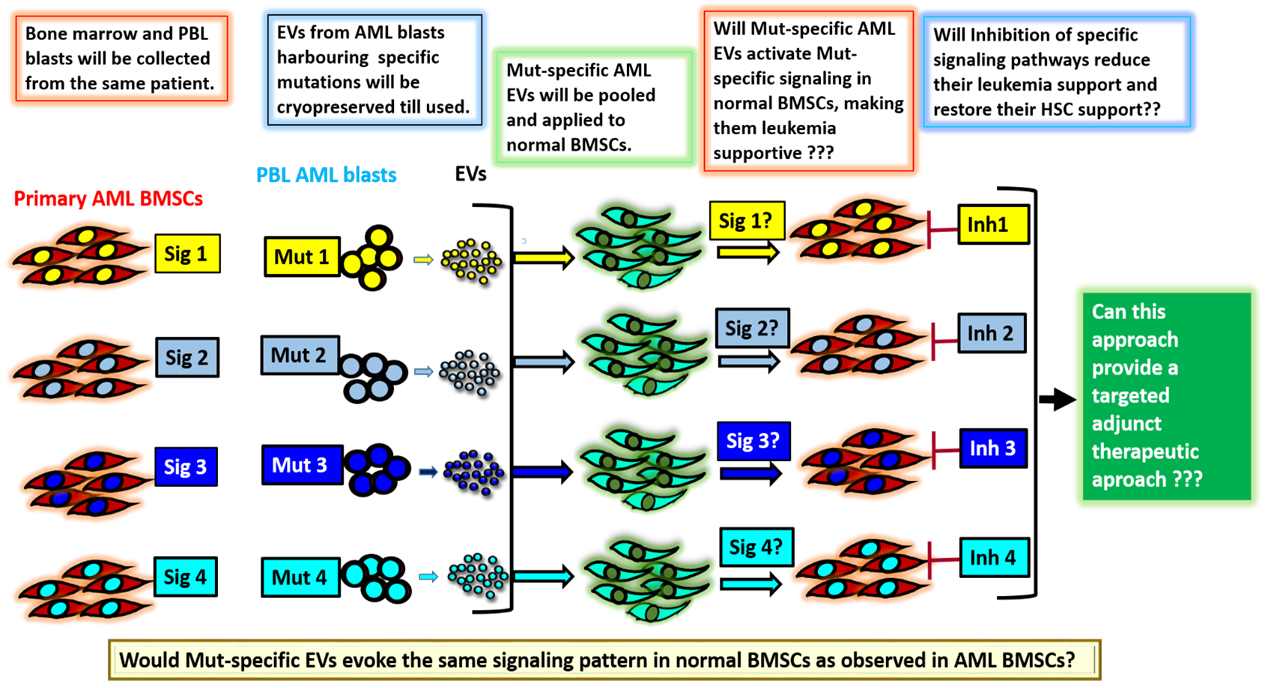
(B) Elucidation of signalling mechanisms induced by EVs secreted by Acute myeloid leukemia blasts having specific mutations
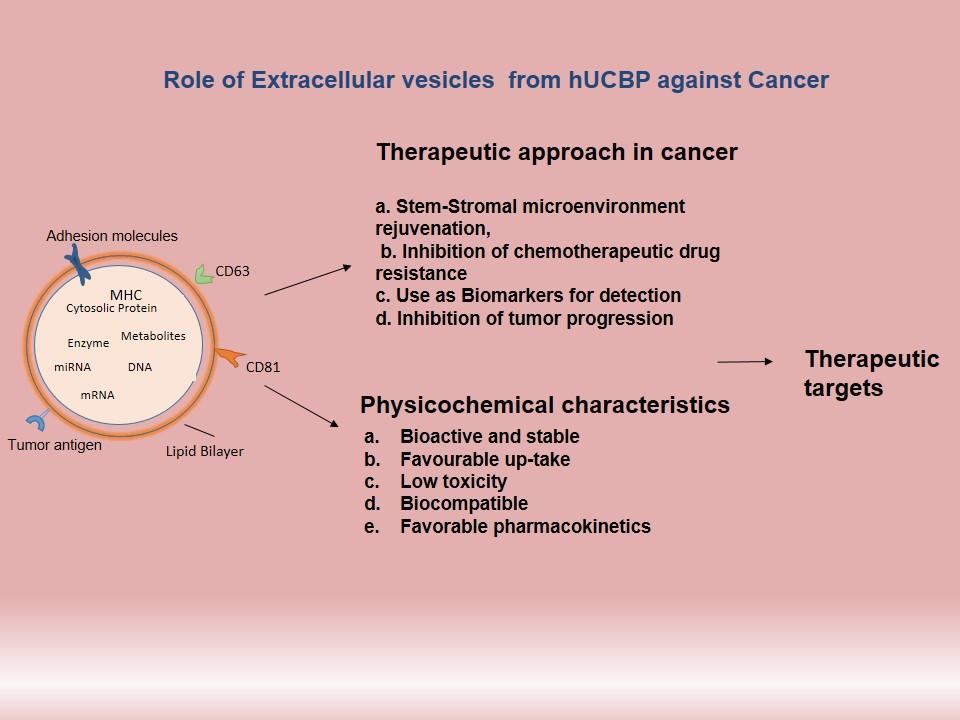
Acute myeloid leukemia (AML) is a type of blood cancer involving specific mutations that can correlate with poor prognosis in patients. In recent years, several studies have underscored the role of the bone marrow microenvironment, especially the MSCs, in the development and progression of the disease. Most of these studies have investigated the effect of the leukemic cells on the bone marrow microenvironment. However, the reverse that is how the leukemic bone marrow stromal cells (BMSCs) modulate the normal HSCs is still not clearly understood. Furthermore, so far, no study has established a correlation between the mutations in the blast cells with the signaling pathways prevailing in the BMSCs. In addition, whether such altered signaling is responsible for conferring a chemo-resistant phenotype on the leukemic stem cells (LSCs), and whether the altered BMSCs lose their HSC- supportive properties has not been investigated. This project focuses on understanding the interplay between the BMSCs and the blasts from AML and aims to decipher how the AML blasts or their extracellular vesicles (EVs) modulate the signaling mechanisms in the marrow stromal cells to their advantage. Such an understanding is crucial because one of the hallmarks of AML is over-production of the immature blasts, which crowd the bone marrow, preventing it from making normal blood cells. Such studies could provide an impetus in designing novel targeted therapies against AML that could be less toxic and more specific.
Principal Investigator: Dr. Anuradha Vaidya and Dr. Vaijayanti Kale
II] Neuroprotective effect of secretome derived from bone marrow-derived MSCs
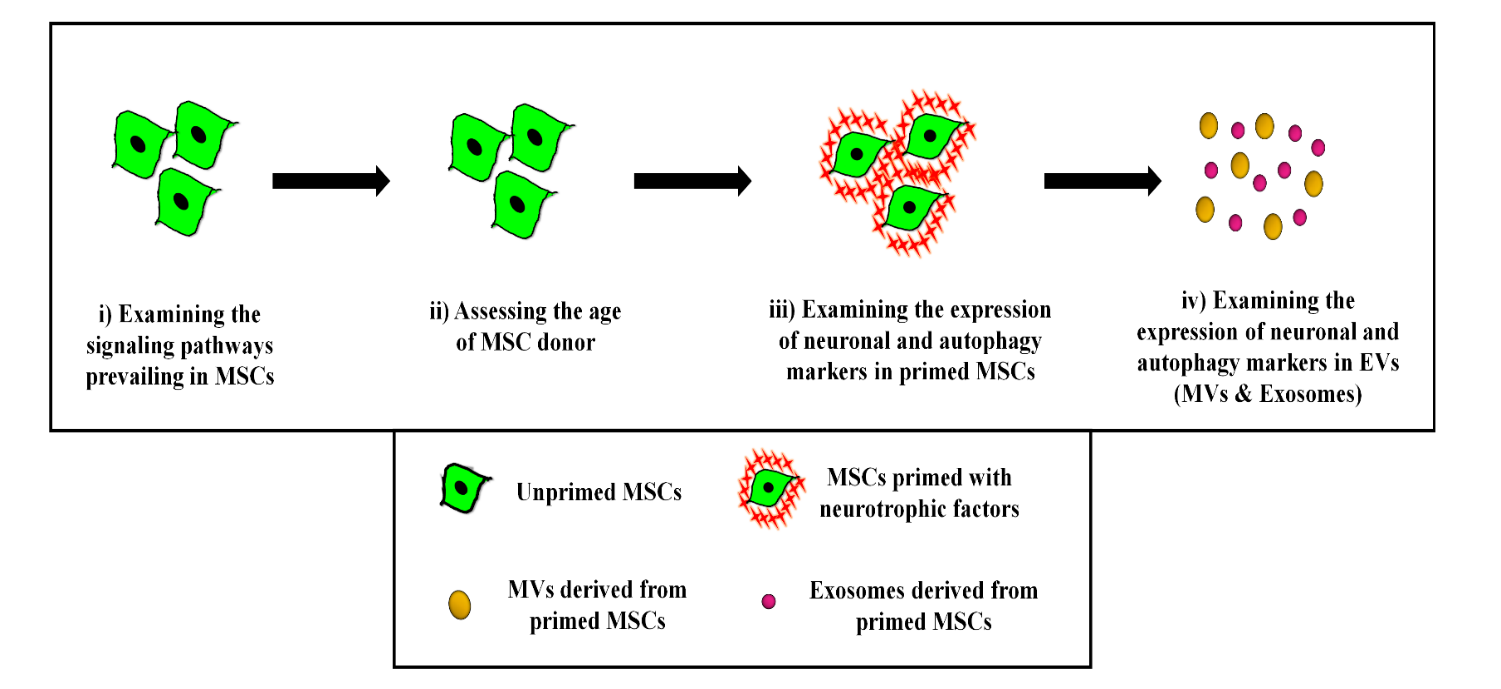
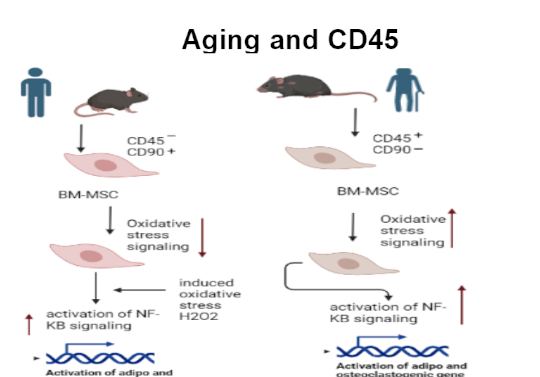
Neurodegenerative diseases (ND) are characterized by a progressive and irreversible loss of neuronal cells leading to cognitive impairments and memory loss. Difficulties in diagnosis and lack of validated in vitro and in vivo models impose challenges in drug discovery and development against ND. Mesenchymal stromal cells (MSCs) are being explored as cell therapy for various ND, but because of their large size they get trapped into primary filter organs like lungs and liver, and also cannot pass the blood-brain barrier (BBB). However, it has been reported that even after being trapped in filter organs, MSCs exert their salutary effects on distant tissues in a paracrine manner through their secretome. Hence, in the last few years, the focus of research has slowly shifted from MSC-based cellular therapy towards harnessing the regenerative potential of the MSCs’ secretome. The primary aim of this project is the establishment of validated oxidative stress and endoplasmic stress-induced-neurodegenerative model systems to examine the salutary effect of the MSC secretome. We would also like to examine whether in vitro priming of young MSCs with neurotrophic factors or certain pharmacological reagents like nitric oxide donors would boost their potential to reverse the loss of neurogenesis induced by stress or aging.
Principal Investigator: Dr. Anuradha Vaidya and Dr. Vaijayanti Kale